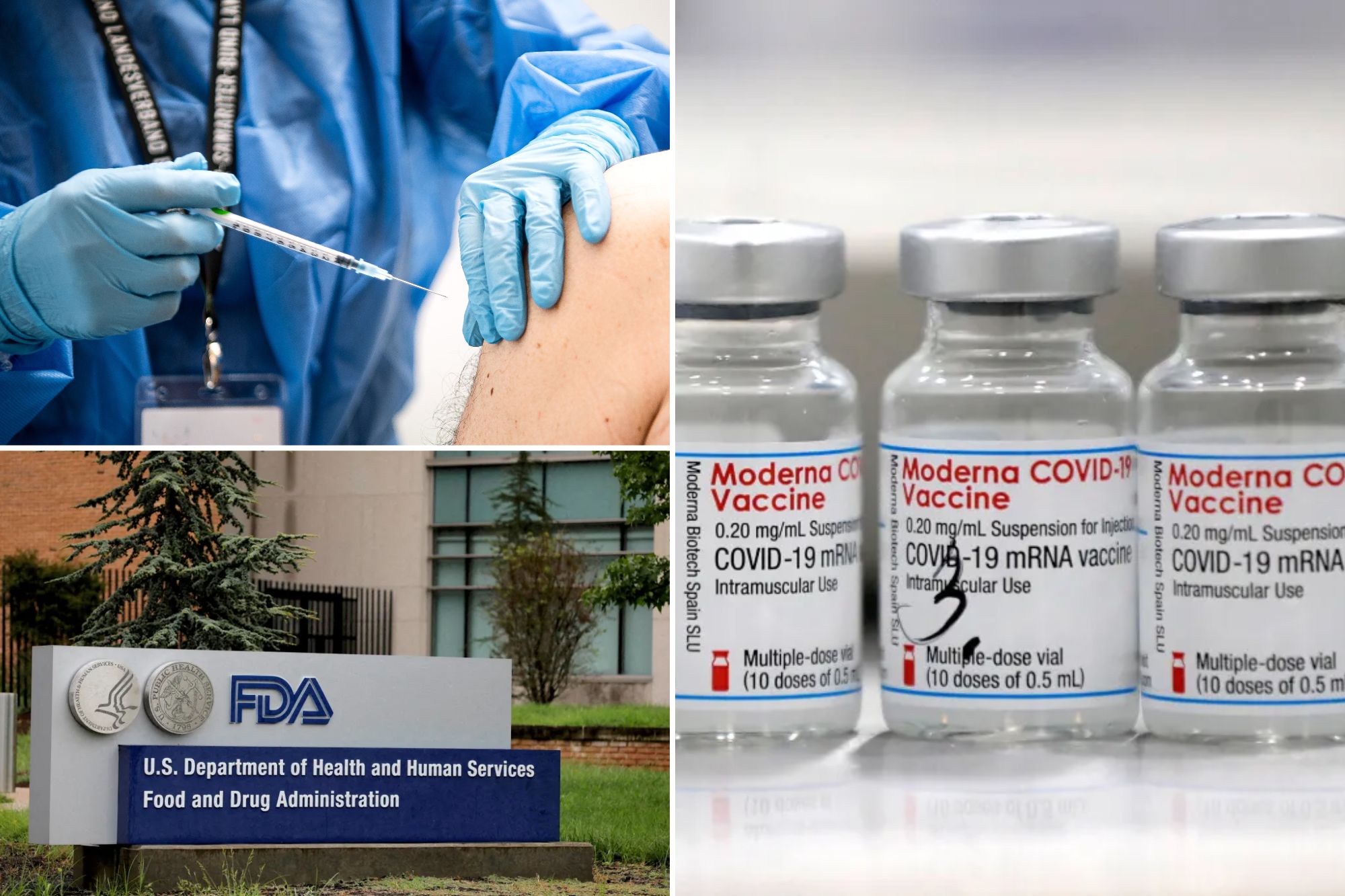
The US Food and Drug Administration has approved the modern Covid-19’s next generation vaccine for all aged 65 and over, the company said on Saturday, the first approval since the regulator tightened the requests.
The vaccine is also approved for people aged 12 to 64 with at least one or more basic risk factors determined by centers for disease control and prevention, Moderna said in a statement.
The company said it expects to have the vaccine, called Mnexspic, available for the 2025-2026 respiratory season.
“The approval of the FDA of our third product, Mnexpike, adds a new important tool to help protect people at high risk of serious illness from Covid-19,” Ceo Stephane Bancel in the statement.
The Department of Health and Human Services, under the leadership of the skeptic of vaccines for a long time Robert F. Kennedy Jr., is strengthening regulatory control over vaccines.
FDA said on May 20 that he planned to ask drug manufacturers to test their Covid booster shooting against a inert placebo in healthy adults under 65 for approval, effectively limiting them to the elderly and those at risk of developing serious illness.
The modern vaccine can be stored in refrigerators than freezer, to provide longer rack life and make distribution easier, especially in developing countries where supply chain issues can inhibit vaccination drives.
Disease control and prevention centers, which Kennedy also supervises, said on Thursday that Covid vaccines remain an opportunity for healthy children when parents and doctors agree that it is necessary, stopping a little from Kennedy notifications days ago that the agency would remove shootings from its immunization schedule.
CDC announcement facilitates the investor’s concern to some extent, say analysts, as it holds the existing framework for the elderly and people at risk that they usually require shots.
FDA leaders have said that 100 million to 200 million Americans would still be eligible for annual shots.
Moderna is betting on her newest Messenger RNA vaccines while dealing with a faded demand for its original Covid Spikevax vaccine and lower than expected of its respiratory syky virus vaccine.
Approval for MNExspic was based on late phase test data, which showed that the goal was not inferior in efficiency compared to Spikevax in individuals aged 12 and older.
The blow also showed superior efficiency compared to Spikevax in adults 18 years of age and older in the study.
Kennedy has launched a large regulation of the health departments, throwing thousands of employees to relate to President Donald Trump’s intention to dramatically reduce the federal government.
This has further ignited concerns about possible interruptions in the regulatory review of treatments and vaccines.
The CDC vaccine external panel in April discussed by recommending reinforcing shooting only to the population at risk of heavy Covid-19 for the next immunization campaign.
FDA approved the Covid Vaccine of Novavax Nuvaxovid this month, limiting its use for the elderly and people over the age of 12 with conditions that endanger them due to the illness.
Conditions that pose an additional risk range from diseases such as diabetes and heart disease to behaviors such as physical inactivity and substance abuse, according to CDC.
While Modern’s shooting, as well as the Pfizer-Bionan COMIRNATY, are based on MRNA, the Novavax vaccine is based on protein and requires more time to produce.
Modern this month withdrew a request demanding approval for his candidate for the flu and covident combination vaccine to expect data for efficiency from a test in the late stage of its flu purpose.
#FDA #approves #generation #modern #generation #adults #older
Image Source : nypost.com